We demonstrated that Interferon regulatory factor (IRF)1 is a component of cyclic dinucleotide recognition that allows mucosal DCs to induce host defense through TH1 7 cells and shape protective immune responses in the intestine to cope with entero-invasive pathogens such as S. typhimurium.
We identified IRF1 as a pivotal transcription factor in the Sensor Stimulator of Interferon Genes (STING) nucleotide sensing pathway responsible for defining the outcome of antigen presentation by mucosal DCs.
We show that STING signaling includes an IRF1 specific transcriptional program that endows DCs with the ability to drive TH17 polarization in the intestine. STING signaling complexes recruited IRF1 and subsequently directed nuclear translocation of IRF1 for the induction of Gasdermin D, IL-1 family member cytokines and enzymes for eicosanoid synthesis.
The identification of the STING-IRF1 signaling axis for adaptive host defense control will aid in the elucidation of disease mechanisms that are initiated by cytosolic host or pathogen DNA processing and recognition.
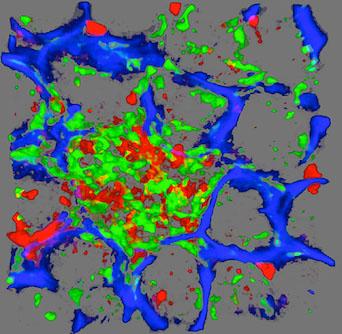
Isolated lymphoid follicle in the small intestine.
Green: CX3CR1+ dendritic cells
Red: CD103+ dendritic cells
Blue: blood vessels.
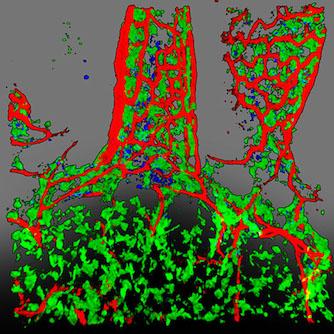
Small intestinal dendritic cell system.
Green: CX3CR1+ dendritic cells
Red: blood vessels