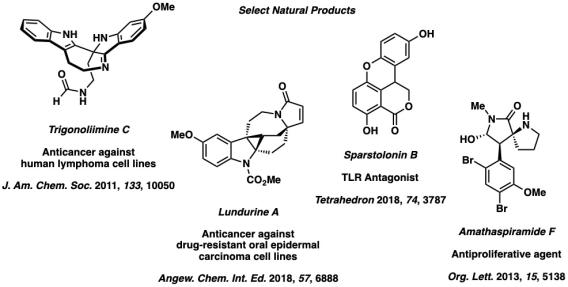
To complement our research in catalysis, we have developed selective methods for the assembly of biologically active polycyclic alkaloids. As part of our natural product synthesis program, select syntheses include synthesis of trigonoliimine C via a selective oxidative rearrangement of a conjugated tryptamine heterodimer and the stereoselective synthesis of the amathaspiramides through our tandem palladium-catalyzed allylic substitution / [2,3]-Stevens rearrangement developed in our laboratory. We have recently completed sparstolonin B through a palladium-catalyzed aldehyde α-arylation and lundurines A and C via a novel vinylogous Pictet-Spengler reaction developed by the Group.