The most rapidly dividing cells in the body reside in the intestinal epithelium, which relies on progenitor cells like intestinal stem cells (ISCs) to sustain themselves. Dietary lipids are known to regulate ISCs, but the mechanisms by which lipids affect intestinal proliferation are not well understood.
Lipid biosynthesis is regulated by sterol regulatory element-binding proteins (SREBPs). Our group’s central goal is to determine the molecular mechanisms by which SREBPs control intestinal epithelial proliferation. We hypothesize that SREBPs maintain homeostasis of intestinal epithelia by producing lipid metabolites that sustain the proliferation of intestinal progenitors.
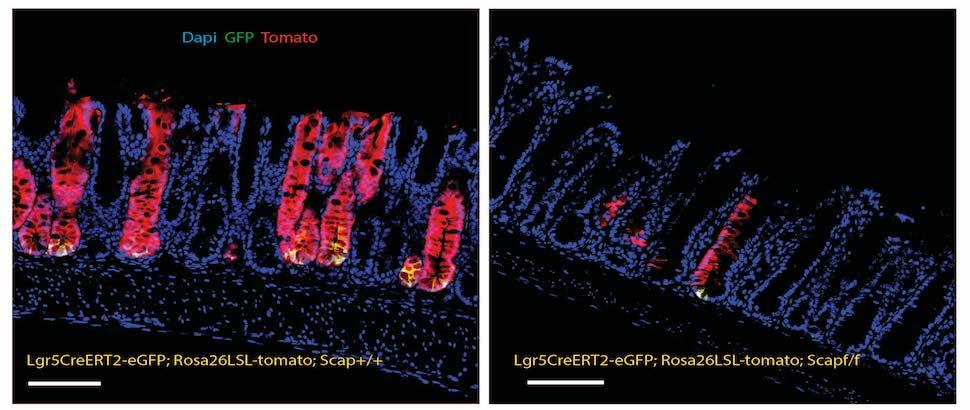
Recent studies from our lab have demonstrated that SREBPs also have a critical role in sustaining the growth of intestinal epithelia. We found, surprisingly, that a deficiency of all SREBPs results in a total collapse of the intestinal epithelia, while selective deficiency of SREBP-2, which selectively blocks cholesterol synthesis, results in marked intestinal overgrowth and increased numbers of intestinal progenitor cells.
Our current projects are:
- To determine how SREBPs drive the growth of intestinal epithelia & ISCs.
- To identify the cellular compartment(s) where SREBPs mediate their effects on intestinal growth.
- Using unbiased transcriptome and lipidome profiling, to identify the lipid metabolites and their biosynthetic enzymes that underlie the effects of SREBPs on the epithelium.
- To determine how SREBP-2, and more broadly the enzymes and intermediates of the cholesterol biosynthetic pathway, control the growth of intestinal neoplasias such as colorectal cancer.
We use novel mouse models using Cre-loxP tools to modulate gene and marker expression in the intestine in vivo, RNA-seq and lipidomics measurements, and intestinal organoids (human and rodent, tumor-derived and from normal intestines) for in vitro mechanistic experiments.
We expect that the major outcome of these projects will be the identification of mechanisms by which SREBPs regulate intestinal growth and tumor formation. It is feasible that the newly identified lipid regulators will represent novel therapeutic targets that could be manipulated pharmacologically. It is also feasible that these novel lipid signals will represent drug targets for the treatment of colon cancer.
Lastly, we hope that a better understanding of lipid metabolism in the intestine will facilitate the development of new treatments for complications of obesity such as non-alcoholic fatty liver disease, which is an epidemic in the U.S.