Pediatric low-grade gliomas (pLGGs) are the most common brain tumors in children, but rarely occur in adulthood. Genetically, pLGGs are a single molecular disease universally driven by abnormal activation of the RAS-RAF-MEK-ERK/MAPK signaling pathway. These clinical observations suggest the existence of a critical developmental window vulnerable to hyperactive ERK/MAPK signaling in the brain. Approximately 10%-15% of pLGGs arise in individuals with neurofibromatosis type 1 (NF1), which harbors germline heterozygous inactivating mutations or deletions in the NF1 tumor suppressor genes, which encodes NF1, a negative regulator of the RAS proto-oncoprotein. NF1-associated pLGGs frequently develop along the optic pathway, also known as the optic pathway glioma (OPG). Although NF1-OPGs does not progress into malignancy, a subset will cause visual impairment and severely impact the quality of life for affected children. Using NF1-OPG as a developmental disease model, we will use gain- and loss-of-function genetic approaches to modulate ERK/MAPK signaling levels to (1) define the developmental dependency of neural stem cells (NSC) in the hypothalamic ventricular zone (hVZ), rendering them vulnerable to ERK/MAPK-driven tumorigenesis, (2) determine the migratory pattern and differentiation of hVZ-NSC-derived glial progenitor cells from the brain to the optic nerve with different levels of ERK/MAPK signaling, (3) elucidate tumorigenic mechanisms caused by the interaction between migrating tumor cells and abnormal immune cells, and (4) perform translational research that develop preventive and treatment therapies for NF1-OPG before tumors cause irreversible neurological and visual impairment.
Brain Development
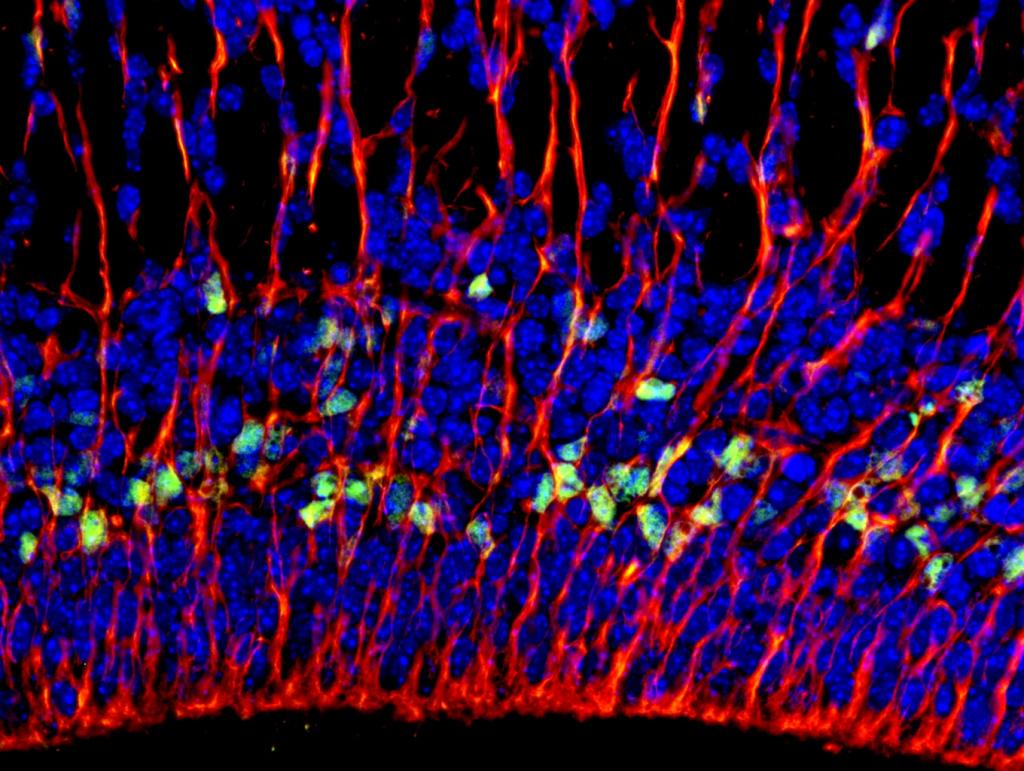
A fluorescent image shows radial glial fibers extending from the ventricular/subventricular zones of the murine neocortex.