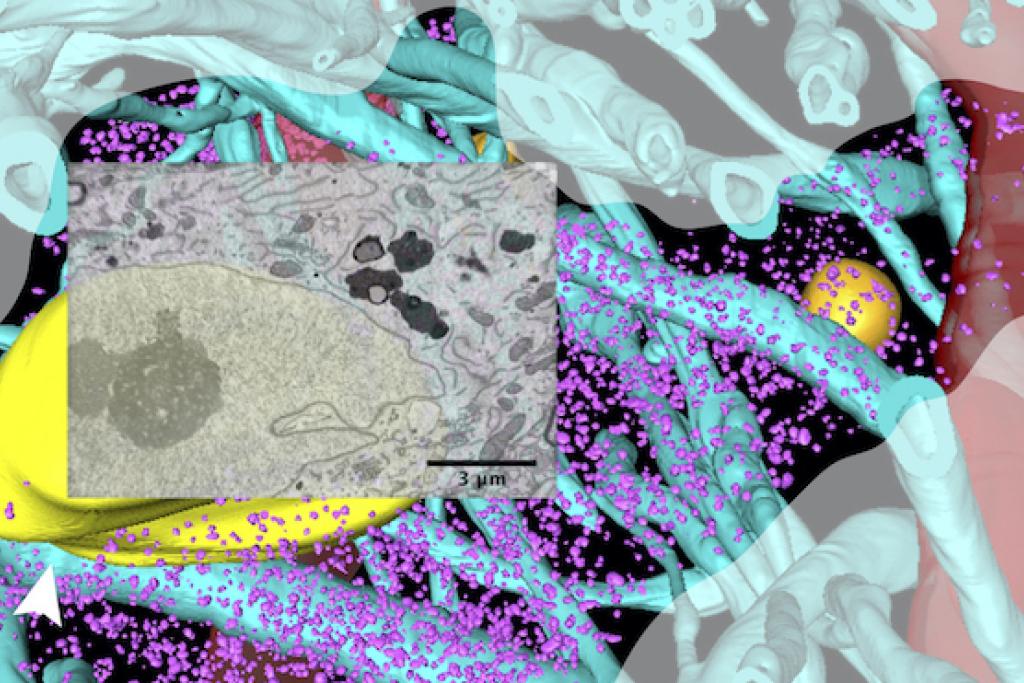
 A neuron in human brain tissue surrounded by axons (blue stalks) and blood vessels (red). Overlaid electron microscopy image shows a window into the neuron's internal structure.
A neuron in human brain tissue surrounded by axons (blue stalks) and blood vessels (red). Overlaid electron microscopy image shows a window into the neuron's internal structure. Investigating disease-causing proteins in their native intracellular setting
Alzheimer’s and Parkinson’s diseases and other neurodegenerative diseases are associated with protein aggregations that arise when proteins misfold and clump into larger, often insoluble structures.
We study the macromolecular basis of how proteins and lipids interact during aggregation: What is their architecture? What precursors do they require? What sub-cellular environment supports them?
What sets our work apart is that we study protein aggregation in situ, which can reveal what these aggregates look like and how they behave in their natural cellular environment.
We developed a neuronal co-culture cryo-platform uniquely compatible for specialized cryo-fixation that preserves cells in a “glassy” state without chemical fixatives and without ice crystals that destroy tissues and cells. This enables correlative light and electron microscopy (CLEM) of near-native neuronal networks.
We harness cellular structural biology techniques and complementary methods. We also develop and apply new interfaces and technologies for cryo-focused ion beam milling (cryo-FIB) and cryo-electron tomography (cryo-ET).
These tools, combined with advanced computation, allows to view nanoscale details inside a cell preserved in a near-native state, either healthy or diseased.
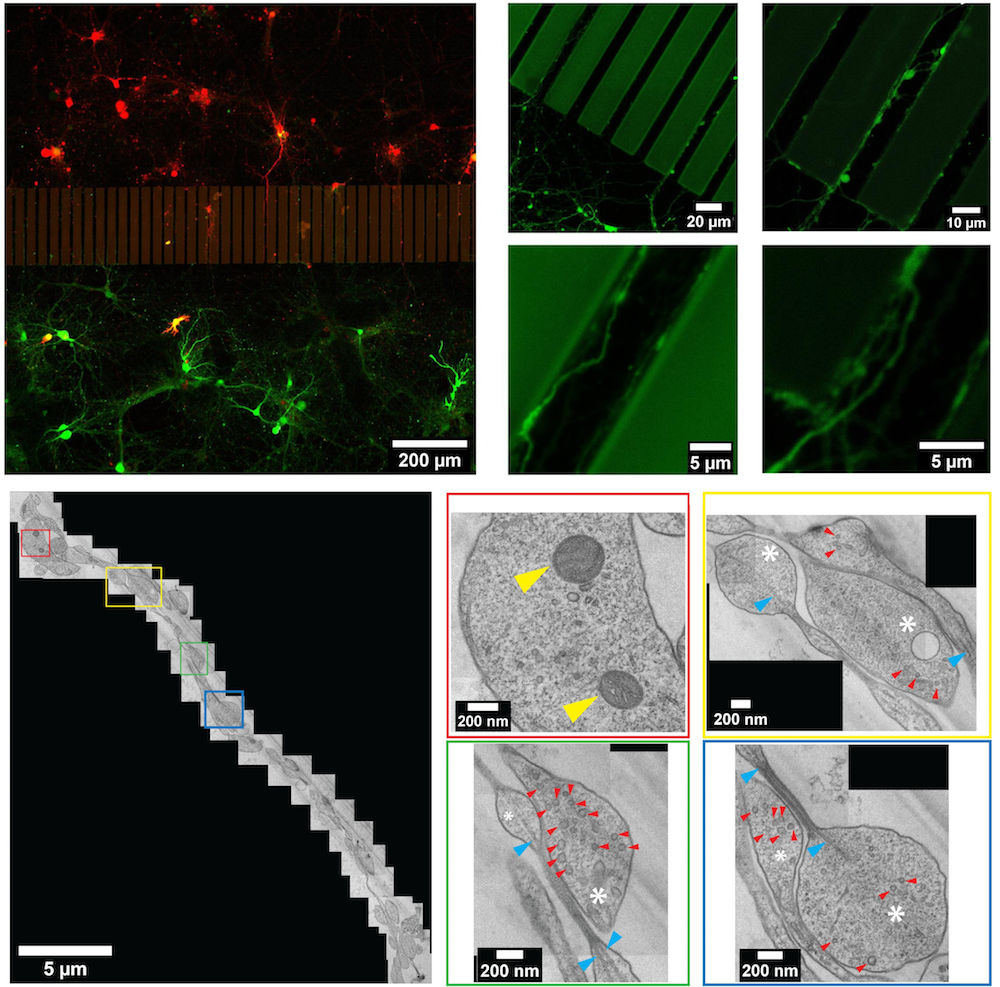 Our microfluidic system for cryo-fixation and CLEM of neuronal networks.
Our microfluidic system for cryo-fixation and CLEM of neuronal networks.  Localizing fluorescently-tagged aggregates within a cellular model of neurodegeneration, using cryo-confocal microscopy.
Localizing fluorescently-tagged aggregates within a cellular model of neurodegeneration, using cryo-confocal microscopy. Our Research Supporters
We are grateful for the generous research funding from the Parkinson's Foundation, National Institute on Aging - National Institutes of Health (NIH), the President's Research Council (PRC), Howard Hughes Medical Institute (HHMI), and the Lyda Hill Foundation.