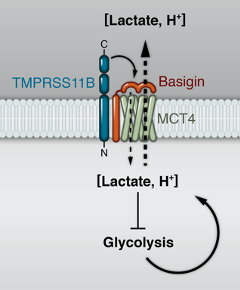
Transmembrane protease TMPRSS11B promotes lung cancer growth by enhancing lactate export and glycolytic metabolism
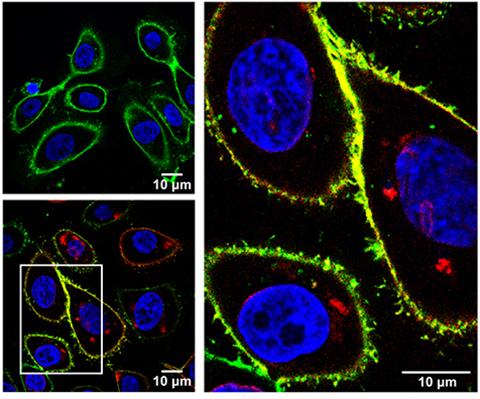
Pathways that drive metabolic reprogramming in cancer are increasingly recognized as critical drivers of tumorigenesis and targets for therapy. We recently performed a transposon-mediated forward genetic screen to identify genes that promote transformation of immortalized human bronchial epithelial cells (HBECs), leading to the discovery that the poorly characterized transmembrane serine protease TMPRSS11B reprograms cancer cell metabolism and acts as a potent lung cancer driver (Updegraff et al., Cell Reports, 2018). TMPRSS11B is upregulated in human lung squamous cell cancers (LSCC) and high expression is associated with poor survival of non-small cell lung cancer patients. Accordingly, TMPRSS11B inhibition in human LSCC cells reduced transformation and tumor growth. Given that TMPRSS11B harbors an extracellular (EC) protease domain, we hypothesized that catalysis of a membrane bound substrate modulates tumor progression. Interrogation of a set of soluble receptors revealed that TMPRSS11B promotes solubilization of Basigin, an obligate chaperone of the lactate monocarboxylate transporter MCT4. A subsequent series of biochemical and metabolomic studies demonstrated that Basigin release mediated by TMPRSS11B enhances lactate export and glycolytic metabolism, thereby promoting tumorigenesis.
These findings contribute to our understanding of cancer metabolism and have important therapeutic implications. First, our data newly establish the tumor-promoting activity of TMPRSS11B, which is highly expressed in LSCC and is associated with poor survival in lung cancer. These results, coupled with the fact that TMPRSS11B is a protease localized to the cell membrane, raise the possibility that this protein may be targeted with novel therapeutic antibodies or small molecules in lung cancer and other malignancies. Second, we linked the transforming activity of TMPRSS11B to its ability to regulate Basigin and lactate export. Finally, there has recently been a resurgence of interest in lactate transport and metabolism, given the discovery that this metabolite is not merely a bi-product of glycolysis but also serves as a fuel in normal tissue and in cancer. This work uncovers an important new component of the machinery that regulates intra- and extracellular lactate levels and links this new pathway to cancer pathogenesis. These studies set the stage for further mechanistic dissection and potential therapeutic targeting of this pathway.