We are interested in understanding the fascinating epigenetic events that lead to transcriptional reprogramming during the development of cancer and other human diseases driven by deregulation of gene expression. We complement mechanistic studies, with genomic and pharmacological approaches, including the discovery of new small molecule epigenetic inhibitors. Come join our exciting projects:
Targeting Jumonji histone demethylases to prevent the development of therapeutic resistance
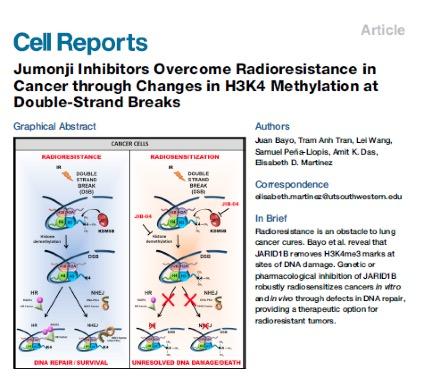
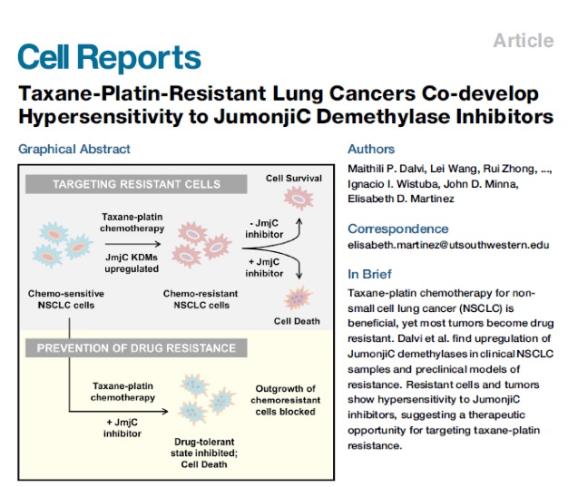
We have that histone demethylases of the Jumonji family of enzymes are essential for transcriptional reprogramming and adaptation to chemotherapy as well as for DNA repair and response to radiotherapy. We are testing approaches to block the onset of therapeutic resistance by inhibiting these enzyme activities in vivo and defining their mode of action.
Defining the connections between cancer cell metabolism and epigenetic outputs and establishing synergy with immune checkpoint blockade
Our group is defining how oncogenic changes in cancer metabolism can lead to new epigenetic vulnerabilities undescribed to date. With the advent and high promise of immune therapy such as checkpoint blockade, we are pursuing epigenetic strategies to brake tolerance in cancer taking advantage of these vulnerabilities.
Genomic and non-genomic functions of Jumonjienzymes in human disease
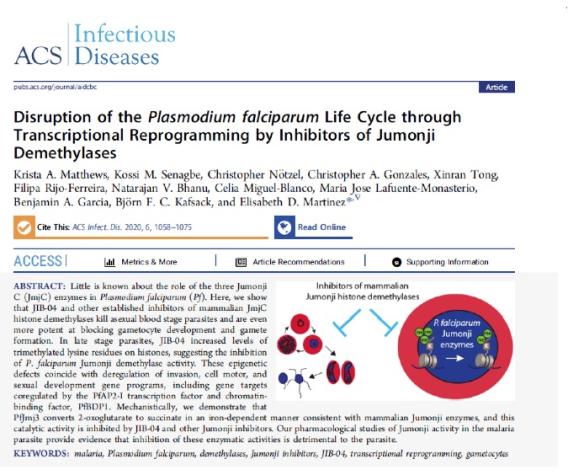
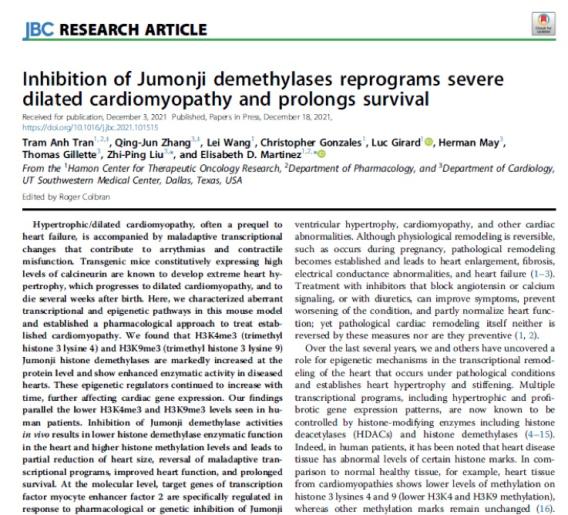
We are finding exciting and intriguing non-canonical roles of Jumonji histone demethylase enzymes in infectious diseases including malaria, HIV, and COVID19, where our data suggests they mediate key aspects of pathogen-host interactions and mediate the pathogen life cycle. We also have found that heart disease relies on Jumonji enzymes to remodel the heart during fibrosis and hypertrophy and our inhibitors block this and restore cardiac health!
Come by and find out more about our research and the open grad student positions! The lab has a very collaborative atmosphere with helpful senior members and stimulating collaborations!