The clinical utility of magnetic resonance spectroscopy and magnetic resonance imaging (MRI) of nuclei such as 13C and 15N has lagged behind 1H MRI largely because of the inherent low sensitivity of detecting these nuclei in tissues.
Liquid-phase dynamic nuclear polarization technology overcomes this low-sensitivity problem by creating nuclear spin-polarization levels that are much higher than room-temperature Boltzman levels.
DNP works by transferring electron-spin polarization from a paramagnetic polarizing agent to coupled nuclear spins by microwave irradiation in the frozen glass matrix. Dramatic 13C and 15N signal enhancements, often more than 10,000-fold, occur after the dissolution of the sample. These enhancements offer the exciting prospect of imaging 13C- and 15N-enriched metabolites in vivo. MR imaging with hyperpolarized nuclei could combine sensitivity approaching that of PET with the resolution of MRI.
We are also interested in the DNP of ultra-low-gamma nuclei such as 89Y and 107,109Ag.
The signal from hyperpolarized nuclei decays by spin-lattice (T1) relaxation. Low-gamma nuclei such as 89Y and 15N usually have very slow T1 relaxation, making hyperpolarized 89Y or 15N attractive as potential in vivo imaging and spectroscopy probes.
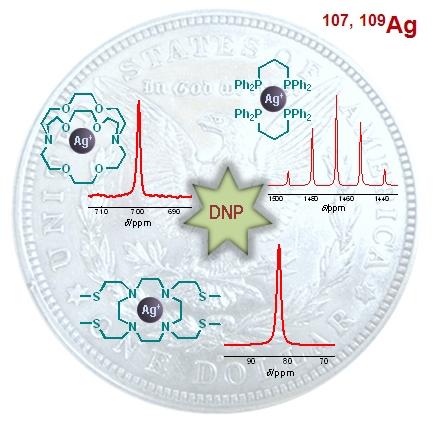 DNP NMR spectra of various silver complexes.
DNP NMR spectra of various silver complexes. 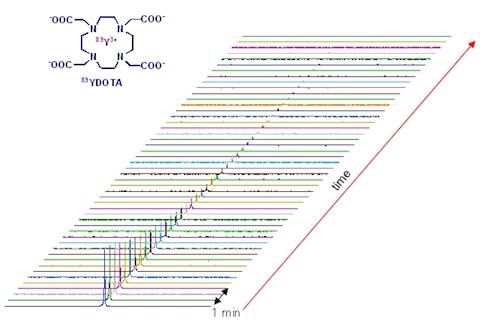 T1 decay of the hyperpolarized magnetization of 89Y-DOTA complex.
T1 decay of the hyperpolarized magnetization of 89Y-DOTA complex.