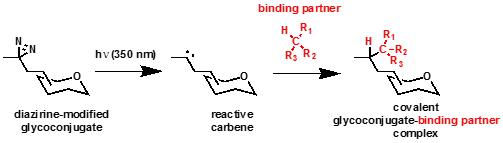
Photocrosslinking of diazirine-modified glycoconjugates to capture glycan-dependent interactions
We have developed photocrosslinking sugar technology that can be used to identify glycan-dependent interactions. We synthesize unnatural analogs of common monosaccharides. The analogs contain the diazirine photocrosslinking functional group. This small, three-membered ring can be activated by UV (~350 nm) irradiation to generate a highly reactive carbene intermediate. The carbene will rapidly insert into nearby C-H, O-H, or N-H bonds, resulting in a covalent crosslink between the sugar and neighboring molecules.
These crosslinked complexes can be purified and/or analyzed by immunoblot or mass spectrometry. Photocrosslinking sugars can be used in purified systems or can be incorporated into the glycoconjugates of living cells, enabling crosslinking of glycan-dependent or -associated interactions in their native, cellular context.
Photocrosslinking sugar analogs of N-acetylglucosamine (GlcNAc) and sialic acid (SiaDAz), as well as related reagents, are available for collaborative research projects.
Funding
SiaDAz and O-GlcNDAzOur research is funded by the NIH (R01GM090271, R21DK112733, and U01CA242115).
Please visit the Common Fund Glycoscience Initiative website for other glycoscience tools under development.