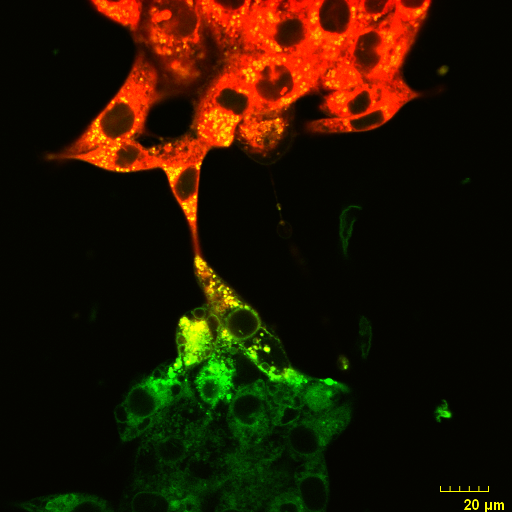
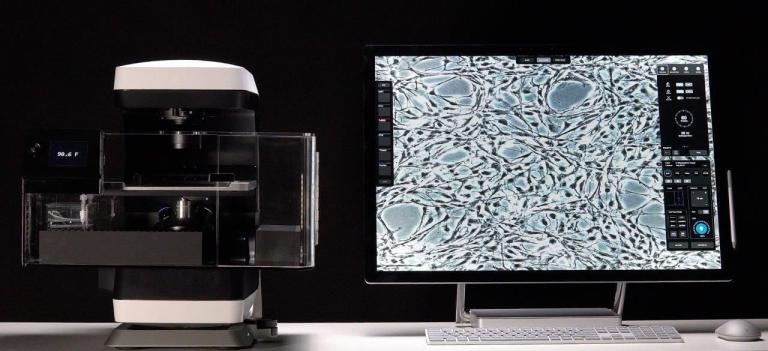
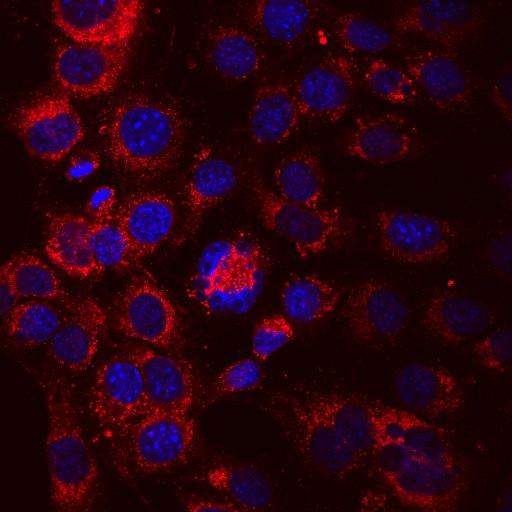
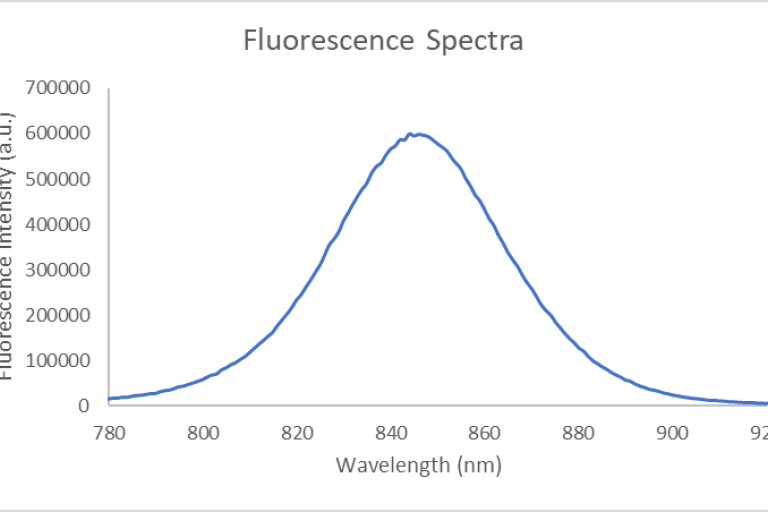
An Olympus FV3000 Confocal Scanning Microscope with Fluorescence Lifetime Imaging
The system consists of a laser scanning confocal microscope (Olympus FV3000) and a set of add-ons from Picoquant to obtain high-resolution, high-contrast fluorescence images (either in intensity and/or lifetime) of a sample. It is specially designed for NIR imaging with ps pulsed lasers and GaAsP/GaAs PMT detectors.
- 4 GaAsP/GaAs PMT detectors
- High Efficiency Spectral Detection using Volume Holographic Gratings
- Conventional XY Glavo and High-speed Resonant Galvo-Galvo Scanning
- Motorized XYZ stage for easy transition between macroscopic to microscopic imaging
- 5 solid-state diode lasers (405nm, 488nm, 561nm, 640nm and 785 nm)
- 4 Olympus Objectives (4X, 10X, 20X and 60X)
- Laser Combining Unit with 5 ps pulsed lasers (405nm, 485nm, 561nm, 640nm and 785nm)
- 2 detection channels for FLIM/FRET with 5 ps minimum bin width
- Picoquant MultiHarp 150 High-Throughput Multichannel Event Timer & TCSPC Unit
Echo Revolution Microscope
- An upright/inverted convertible wide-field microscope with an incubator for live cell imaging.
- The system has four objectives (4X, 10X, 40X and 60X) and four channels of fluorescence detection (DAPI, FITC, CY5 and CY7).
- Auto-focus and image stitching make it a very user-friendly tool.
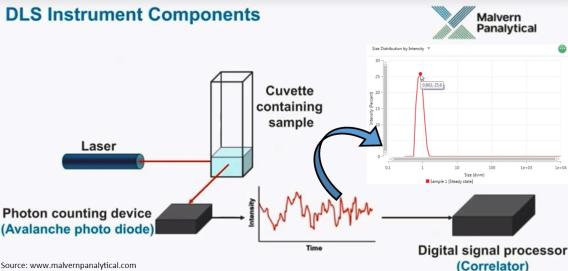
Dynamic Light Scattering
Dynamic Light Scattering (DLS) is one of the most convenient and well-known techniques for measuring the size of different nanoparticles and nanocomposites dispersed in liquid suspension in sub-micron size range. DLS captures the fluctuations in light scattered by suspended particles. These fluctuations in the scattered signal are directly correlated to the Brownian motion of the particles and, thereby, the size of nanoparticles. Our Zetasizer Lab series from Malvern Pananalytical (UK) is also capable of measuring the size distribution, average particle size, and zeta potential.
|
Cube LED Description |
Cube Filter (Excitation) |
Emission |
|
DAPI |
377 nm |
447 nm |
|
GFP |
469 nm |
527 nm |
|
Cy5 |
628 nm |
685 nm |
|
Cy7 |
716 nm |
809 nm |
Cytation 5 Imaging Plate Reader
The BioTek Cytation 5 imaging plate reader has a widefield microscope capability using high-powered LED cube-filter cube assemblies, including a NIR Cy7 channel.
Imaging protocol: Endpoint, time-lapse, z-stack, and montage imaging method
Objective lens: Six microscopes’ objective capacity. 4X, 20X and 60X are available.
1.25x to 60x magnification to cover broad imaging applications.
Camera: 16-bit camera for a dynamic range of > 65,000 fluorescence units
Autofocus, autoexposure, auto-LED intensity adjustments.
Gene 5 software: Image analysis software for image processing, automatic cell counting, and subpopulation analysis. Image processing, including stitching, z-projection, and digital phase contrast.
Applications: (1) Reader- Absorbance, fluorescence, and luminescence available. Other potential applications are fluorescence polarization and (2) Microscopy: - Four microscopy and fluorescence reading color channels with PMT extended red. Other potential applications include a Gas controller for CO2 and O2, a Dual reagent injector for automatic dispensing, and temperature control up to 65°C.
NIR I & II Fluorometer
The custom Horiba Jobin Yvon fluorometer is based on the FluorologQM-75-11-C framework. The fluorometer maintains two detectors: the R13456 PMT (185-900 nm) for UV/Vis/NIR-I spectroscopy and the DSS-IGA020L/ CUS liquid N2-cooled InGaAs detector (800-1700nm at ambient temperatures; 800-1550nm at LN2 temperature of -196 C) for performing spectroscopy in the NIR-I/-II spectral regions. Gratings are blazed at 750 nm and 1000 nm (600 l/mm density). The instrument also features 2 pulsed laser diodes (set at excitation wavelengths of 785 nm and 670 nm) for performing fluorescence lifetime measurements. In addition, the fluorometer is also equipped with a 3.2” integrating K-Sphere for obtaining absolute quantum yield measurements in solid, liquid, or powder form).
Lambda 1050+ Spectrophotometer
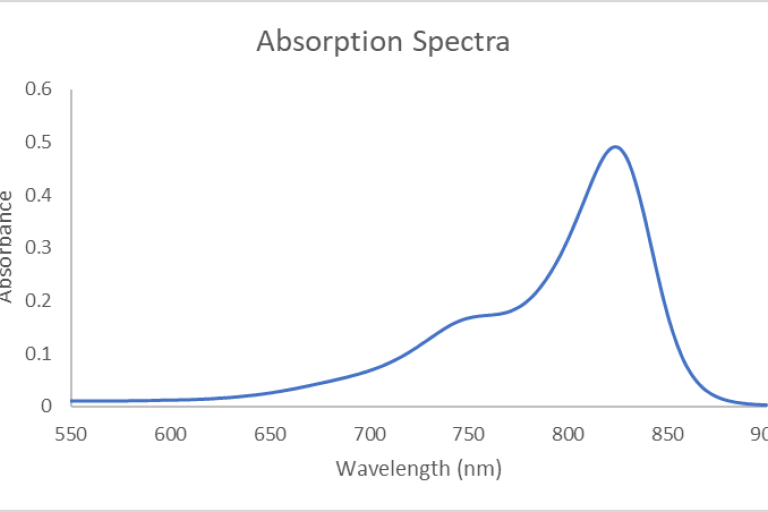
"The lambda 1050+ spectrophotometer measures absorbance (or transmittance) in solution with an operational range of 175 nm to 3300 nm through its three detectors. The photomultiplier tube R6872 (PMT) detector covers the UV/Vis range of 175 nm-860 nm with a resolution of ≤0.05 nm, whereas the indium gallium arsenide (InGaAs) and the lead sulfide (PbS) detector cover the NIR range of 860 nm-3300 nm with a resolution of ≤0.20 nm. The sources are pre-aligned deuterium and tungsten-halogen lamps, which utilize a source-doubling mirror for greater sensitivity to characterize samples accurately. The spectrophotometer comes with the UV Win Lab software, which is operationally simple and easy to use for measurement and analysis."
(Images: PerkinElmer website)
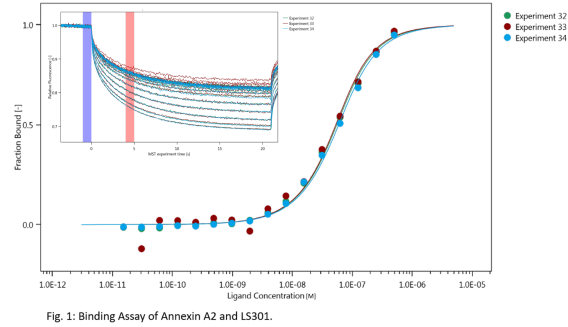
Thermophoresis
The machine is equipped with the MO.control software that provides step-by-step instructions for sample preparation and data quality assessment tools for pretest, binding check, and binding affinity experiments. The machine is also equipped with the MO. Affinity Analysis software for data analysis. The thermophoresis machines (Monolith NT.115pico) in our lab are equipped with the following detectors:
Pico RED detector:
- Excitation wavelength: 600-650nm.
- Fluorophore concentration ≥ 50 pM.
- KD range: pM-mM.
- Sample fluorophores: NT650, Alexa647, and Cy5.
Nano BLUE detector:
- Excitation wavelength: 460-490 nm.
- Fluorophore concentration ≥ 5 nm.
- KD range: nM- mM
- Sample fluorophores: fluorescein, GFP, and AlexaFluor488.
The key features of the instrument include:
- Fast measurement of kD in about 10 min.
- Measurement of wide kD range from pM/nM-mM.
- Low sample consumption: sample volume (≤10µL per concentration).
- Immobilization free, in-solution measurements.
- Measurement in complex mixtures (cell lysates, serum, detergents, liposomes).
- Wide size range of interactions (from ions to molecular dimers).
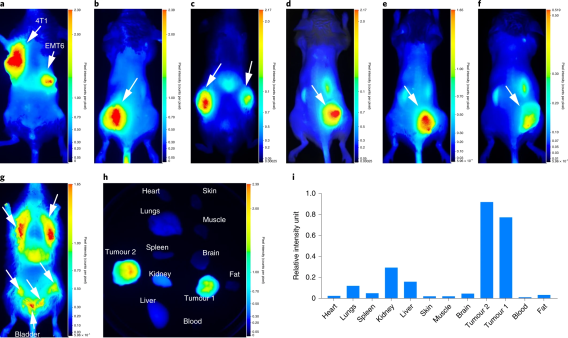
Pearl small animal imaging
The Pearl® Imaging system employs a CCD-based optical system specifically designed for in vivo imaging in the near-infrared (NIR) spectral region where autofluorescence and light scattering are low. The NIR laser illumination also achieves deeper tissue penetration, which permits visualization of smaller targets embedded deeper in tissues. The instrument allows scanning times of 30 seconds or less, minimizing animal stress and increasing investigator productivity. The instrument can be used for performing non-invasive imaging of anesthetized animals in vivo to evaluate a variety of biological variables related to disease, the activity and distribution of drugs, and the effects of drugs. It is also capable of imaging over dual channels, allowing multiple targets to be sampled in the same subject.
System Specifications:
- White Filed Imaging
- 700 Channel Laser Source: 685 nm
- 800 Channel Laser Source: 785 nm
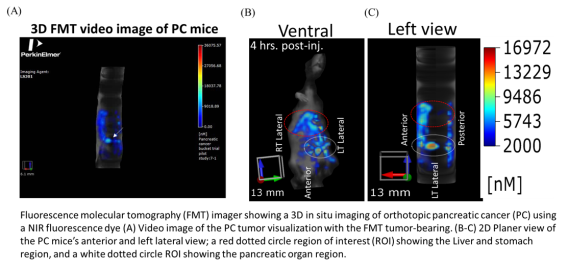
FMT Fluorescence Tomography in vivo Imaging Systems
The FMT® (Fluorescence Molecular Tomography) system is a small-animal fluorescence in vivo
imaging system for murine preclinical research use. It is designed to provide calibrated quantitative
tomographic images and data of fluorescence signals within biological tissue throughout the depth of
the subject. The system is also upgradeable to four imaging channels (FMT 4000).
System Specification:
- Two near-infrared channels at 670 nm and 746 nm, and emitting at 700 nm and 775 nm respectively
- The two additional channels excite at 635 nm and 790 nm and emit at 660 nm and 805 nm respectively
eXplore Optix system
This instrument was developed by ART Advanced Research Technologies Inc., Montreal, Canada, and is used in imaging the small animal in the time-domain fluorescence lifetime distribution.
The interest in fluorescence imaging has increased steadily in the last decade. Using fluorescence techniques, it is feasible to visualize and quantify the function of genes and the expression of enzymes and proteins deep inside tissues. When applied to small animal research, optical imaging based on fluorescent marker probes can provide valuable information on the specificity and efficacy of drugs at reduced cost and with greater efficiency. Meanwhile, fluorescence techniques represent an important class of optical methods being applied to in vitro and in vivo biomedical diagnostics, towards noninvasive clinical applications, such as detecting and monitoring specific pathological and physiological processes. ART has developed a time domain in vivo small animal fluorescence imaging system, eXplore Optix. Using the measured time-resolved fluorescence signal, fluorophore location and concentration can be quickly estimated. Furthermore, the 3D distribution of fluorophore can be obtained by fluorescent diffusion tomography. To accurately analyze and interpret the measured fluorescent signals from tissue, complex theoretical models and algorithms are employed. We present here a numerical simulator of eXplore Optix. It generates virtual data under well-controlled conditions that enable us to test, verify, and improve our models and algorithms piecewise separately. The theoretical frame of the simulator is an analytical solution of the fluorescence diffusion equation. Compared to existing models, the coupling of fluorophores with finite volume size is taken into consideration. Also, the influences of fluorescent inclusions to excitation and emission light are both accounted for. The output results are compared to Monte-Carlo simulations.